Home > Ventilación invasiva
Ventilación invasiva
Ventilación invasiva
¿Qué es la terapia de ventilación invasiva?
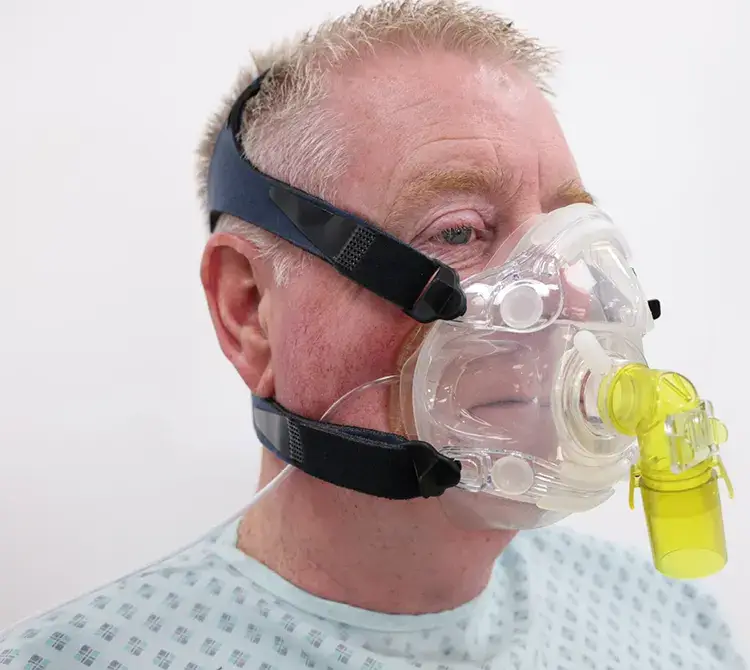
La asistencia ventilatoria invasiva es una parte importante del tratamiento clínico en el ámbito de los cuidados intensivos. Para ayudar a los pacientes a respirar, puede ser necesaria una vía aérea artificial y un soporte respiratorio completo, que debe administrarse con gases calentados y humidificados para aumentar la comodidad del paciente y reducir el riesgo de infección.
Los beneficios de esta terapia incluyen: defensa contra las infecciones, ventilación eficaz y reducción del esfuerzo respiratorio.

Defensa contra las infecciones
Una humidificación óptima ayuda a proteger al paciente contra las infecciones.

Ventilación eficaz
La humidificación de la terapia de ventilación ayuda a reducir la acumulación de secreciones y facilita la ventilación.
Reducción del esfuerzo respiratorio
La terapia de entrega y las estrategias de desconexión se ven favorecidas por la humidificación.
Los circuitos de ventilación invasiva de Eakin Healthcare ayudan al personal de enfermería a administrar la terapia óptima lo antes posible y a mantenerla de forma continua en beneficio del cuidado del paciente.
Los beneficios de esta terapia incluyen: Rama espiratoria seca, humidificación óptima y protección antimicrobiana
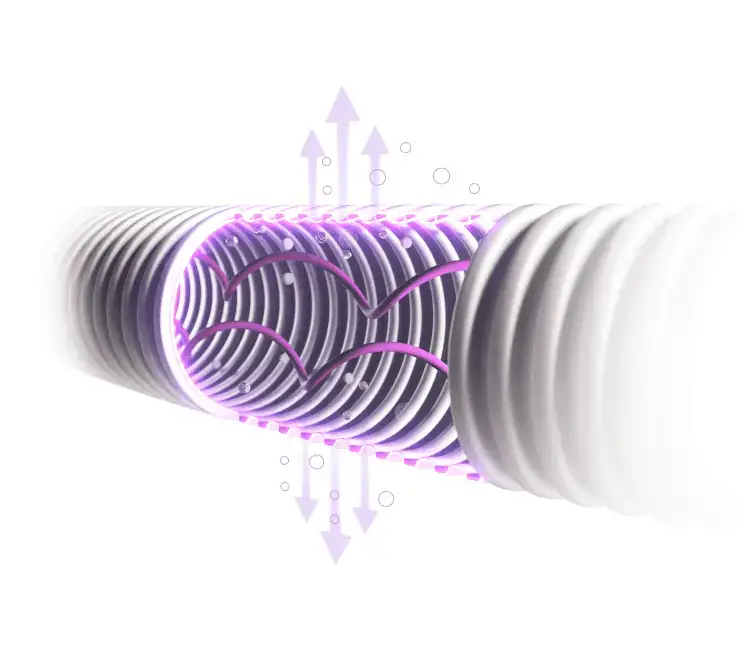
Rama espiratoria seca
La tecnología de transmisión de vapor presente en la rama espiratoria reduce el riesgo de condensación.
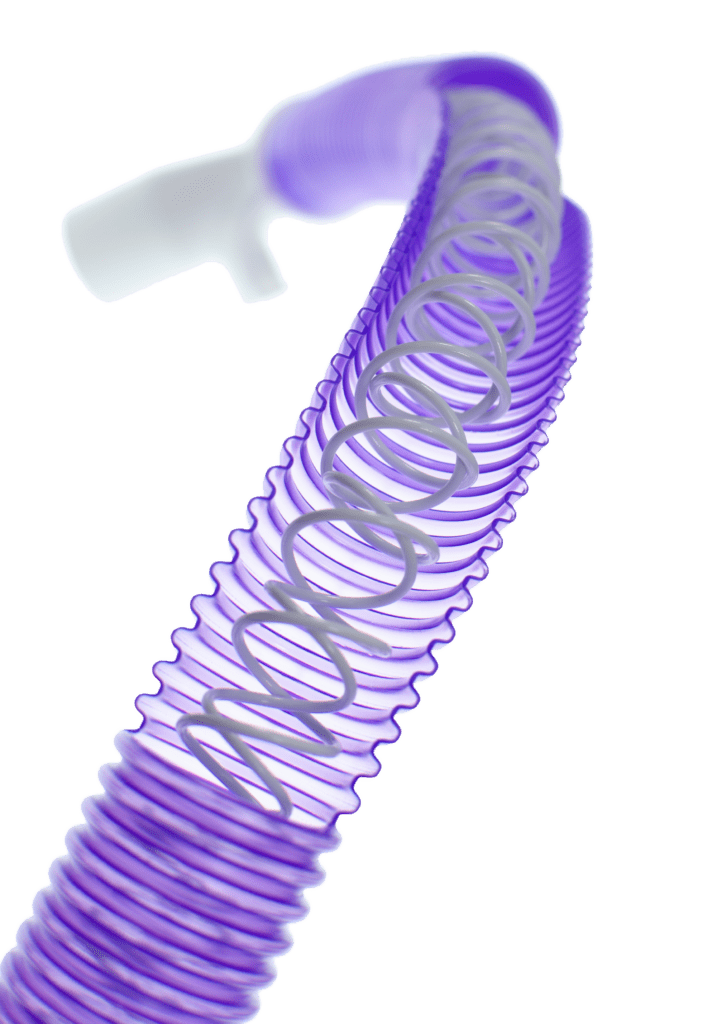
Humidificación óptima
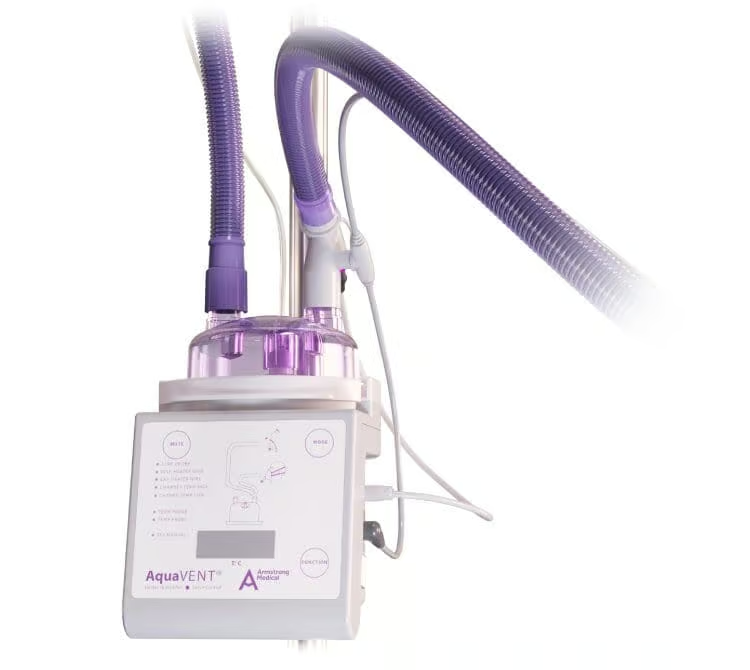
El circuito AquaVENT® VT utiliza la tecnología de humidificación Passover para proporcionar el nivel óptimo de humidificación en beneficio del paciente.

Protección antimicrobiana
Nuestros circuitos de ventilador AquaVENT® VT cuentan con la protección BioCote®, que ayuda a reducir el riesgo de infección.
Preguntas frecuentes
¿Durante cuánto tiempo podemos utilizar el circuito?
El circuito AquaVENT® Vt se puede usar durante 14 días en un solo paciente.
¿Este circuito es compatible con todos los ventiladores?
Sí. El circuito AquaVENT VT® se puede conectar a todos los ventiladores mediante conexiones de 22 mmF.
Circuitos de ventilador humidificado calefactado AquaVENT® VT
Referencias
[1] Pinto, Venessa L., and Sandeep Sharma. «Continuous positive airway pressure.» StatPearls [Internet]. StatPearls Publishing, 2021.
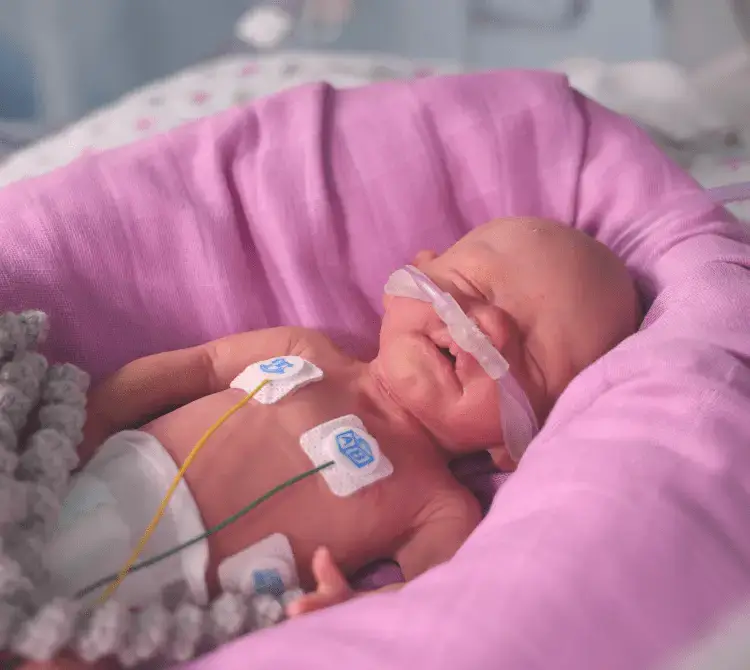
Nuestros circuitos de ventilación NeoFlow® se fabrican pensando en el bebé, el personal médico y la familia. Nuestros circuitos de ventilador NeoFlow® VT incorporan la tecnología de «transmisión de vapor» que crea una rama espiratoria seca que reduce el riesgo de lluvia y mejora la facilidad de uso y la seguridad del circuito.
Los beneficios de esta terapia incluyen: rama espiratoria seca, pieza en Y con triple giro y protección antimicrobiana
Rama espiratoria seca
La tecnología de transmisión de vapor presente en la rama espiratoria reduce el riesgo de condensación.

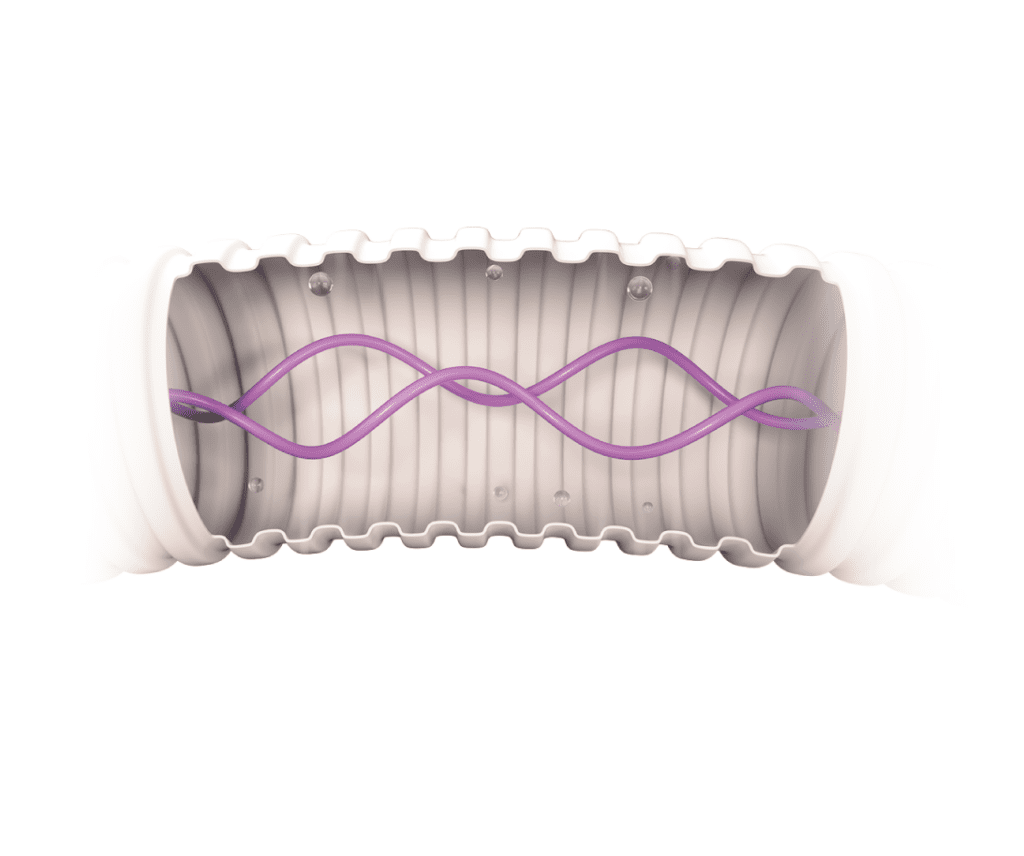
Pieza en Y de triple giro
Nuestra exclusiva pieza en Y para el paciente gira en 3 puntos para ayudar a recolocar al bebé y aliviar la tensión del circuito mientras se mantiene la terapia continua.

Protección antimicrobiana
Preguntas frecuentes
¿Con qué ventiladores podemos utilizar NeoFlow® VT?
Nuestros circuitos son compatibles con todos los ventiladores estándar. (Para consultas específicas sobre la compatibilidad de ventiladores, póngase en contacto con nosotros)
¿A dónde va el agua condensada si no se acumula en la rama espiratoria?
Nuestra tecnología especial de transmisión de vapor permite que las moléculas microscópicas de vapor de agua salgan del tubo a través de vías permeables en la estructura de la pared del tubo. De esta forma, se liberan a la atmósfera fuera del circuito.
¿Es peligrosa la adición de BioCote® para mi paciente neonatal?
No. BioCote® es un ingrediente que añadimos durante el proceso de fabricación. Al ser parte integrante de la estructura del producto, no puede desplazarse ni perjudicar al bebé en modo alguno.
Póngase en contacto con nosotros
Nos comprometemos a proteger y preservar la privacidad de nuestros visitantes siempre que accedan a nuestra página web o se pongan en contacto electrónicamente con nosotros.